Get Certified
If you are interested in learning more about the certification program or would like to request a copy of the application, please contact Bella Shain at [email protected].
Overview
Certified Biostimulant is based on the United States Biostimulant Industry Guidelines, which provides criteria for the proper documentation of evidence to support efficacy testing methods, composition, and safety. The program provides several benefits for manufacturers, ag retailers, and growers. Companies with approved products will be provided a label to utilize on their plant biostimulant, which can be helpful for consumer branding and ag retailers’ confidence.
The Benefits of Being Certified
Certification is a value-add for everyone
Manufacturers
Demonstrates conformance with fair and equitable voluntary standards to show products meet efficacy, composition, and safety attributes.
Ag Retailers
Enables ag retailers to assess products more confidently for shelf space, assists in the selection of products for investing in further independent field trials, and ultimately grower recommendations.
Growers
Allows farmers to make informed decisions based on a recognized industry program.
How to Get Certified
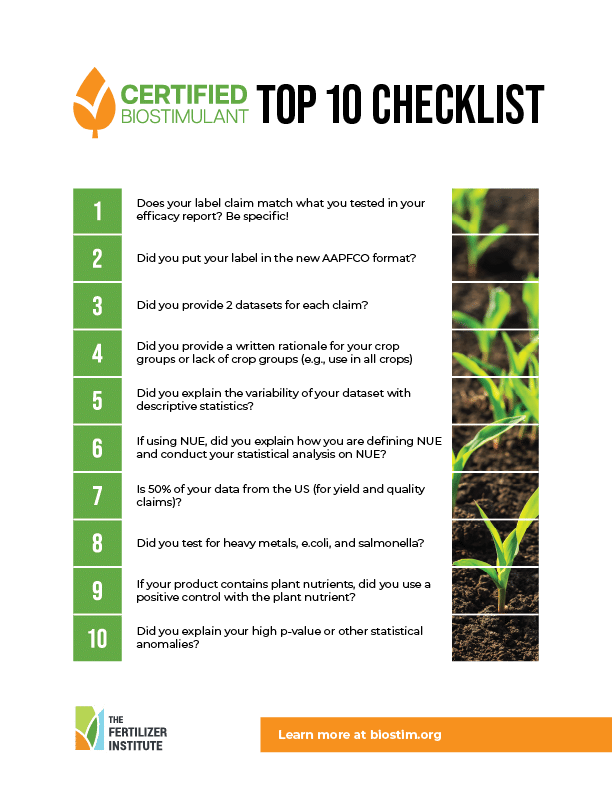
The Certified Biostimulant is aligned with requirements found in United States Biostimulant Industry Recommended Guidelines to Support Efficacy, Composition, and Safety of Plant Biostimulant Products.
STEP
Completion of application including proof of conformance and payment
STEP
Conformance review by independent experts
STEP
Certification Award
Karl Barnhart, Brandt
As a TFI member, you’ll get support as you advance in:
Seeding Success
Improve your bottom line with critical market intelligence to help you remain competitive.
Industry Image
Get guidance and tools to tackle tough issues and benefits from TFI’s 4R Nutrient Stewardship program.
Cultivating Community
Gain insight from real-world evidence and valuable stories from the field as you connect with peers.
Active Advocacy
TFI champions the industry, shapes policy, and delivers resources that help you navigate the regulatory environment.
Certified Biostimulant FAQs
Answers to some of the most-asked questions
Certified Biostimulant is a voluntary program that certifies that information to support label claims of a biostimulant product conforms to the United States Biostimulant Industry Guidelines to Support the Safety, Composition and Efficacy of Plant Biostimulant Products. It provides a consistent framework for reporting a minimal dataset on safety, composition, and efficacy tests conducted on the product. The program assures the existence of such data without disclosing the results. The program certifies that proper experimental protocols were used to test efficacy, without making any assertion on the efficacy of the product for any claims made.
While the program does not require or ask for confidential information, TFI has policies in effect to protect any submitted data. However, a web portal of certified products containing the company name; company website, product name; product category (i.e. one of the 5 product types identified in the standard), sales staff contact name, email and phone number; certification expiration date; certification identification (ID) number; link to the product label; label claims; SDS; and the full efficacy workbook (or CBI redacted information efficacy workbook.) The information appearing on the TFI website is a marketing and promotion tool for the companies that participate. TFI wants to highlight and promote companies in the program as a benefit of participation. It should be noted that all expired certified products will be removed from the web portal.
There will be two fees to participate – an application submittal fee and a renewal fee. The
renewal fee occurs every 2 years after the initial application is submitted.
- Members
o Application fee: $1,800 USD/product
o Renewal fee (every 2 years; no changes to product): $750 USD/product
o If there are any changes to the product, rather than a renewal, the company must re-submit the product Application and Application Fee($1,800 USD).
- Non-Members.
o Application fee: $5,500 USD/product
o Renewal fee (no changes to product) $1,500 USD/product
o If there are any changes to the product, rather than a renewal, the company must re-submit the product Application and Application Fee ($5,500 USD). - The certification identification will list a 6 year expiration date. There are to be attestations from biostmulant manufacturers on product claims and formulation changes every two years. Modifications to product active ingredients constitute a formulation change. Any reported changes will suspend the current certification until a new application is submitted and approved.